 欢迎来到上海谷研实业有限公司网站!
欢迎来到上海谷研实业有限公司网站! 咨询电话:18321818584
咨询电话:18321818584 欢迎来到上海谷研实业有限公司网站!
欢迎来到上海谷研实业有限公司网站! 咨询电话:18321818584
咨询电话:18321818584istological Analys s
Mouse tumors were sectioned and stained for immunofluorescence. After staining, images were obtained using a confocal microscope. Subcutaneous tumors collected from C3H mice were embedded with OCT, frozen for cryosec tions, and stained with filipin III (Guyan, Shanghai, China) at room temperature, and rinsed with PBS to determine the tumor cholesterol levels. The images were acquired using a confocal microscope.
Tumor Suppression and Immune Response in vivo
To establish an oral squamous cell carcinoma animal model, wild-type 6-to 8-week-old female C3H mice procured from Vital凡ver Laboratory Animal Technology (Beijing, C血a) were subcutaneously injected with 3x106 SCCVII cells in the right armpit. The murine SCC cell line SCCVH was provided by Professor Yixiang Wang, Peking university school of stomatology. The 25 mice were randomly divided into five groups (5 animals per group), followed by intravenous injection of 1x PBS (200
µL) for groups (a) and (b), cholesterol for group (c), and GDYO (200 µL, 1 mg/mL) for groups (d) and (e). In group (e), intratumoral injections of cholesterol (2 mg per mouse) were administered to post-PDT animals every other day until their sacr币ce. During treatment, the tumor area ((length x width2)/2) and body weight were monitored every two days.
The mice were euthanized, and the tumors were fixed for immunofluorescence. Tumors and draining lymph nodes
(DLNs) were isolated from all five groups and prepared as single-cell suspensions using digestive enzymes. Cells were stained with fluorescence-labeled anti-CD45, anti-CD3, anti-CD8, anti-IFN-y and anti-TNF-a antibodies (eBioscience, USA), and dendritic cells (DCs) were tagged with anti-F4/80, anti-COi Jc, anti-CD80, and anti-CD86 antibodies (eBioscience, USA), and then subjected to flow cytometry.35 The Institutional Animal Care and Use Committee (IACUC) at Sun Yat-Sen University (Approval Number: SYSU-IACUC-2020-00042) reviewed and approved the animal use for these experiments, and all of the animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals.
Statistical Analysis
All data are expressed as mean士standard deviation (SD). Data graphs were obtained using Prism 8.0. P <0.05 was considered statistically significant.
Results and Discussion
GD丫0 Characterization
As a novel material, the synthesis of GDYO was mainly performed by the acid-oxidation treatment. There are two main acid oxidation ways to produce GDYO: the conventional Hummers'method and the modified Hummers'method. In the conventional Hwruners'method, the widely used acid was a mixture ofH2S04 and KMn04. Compared with the conventional Hummers'method, The distinctive feature of the modified Hummers method is the choice of different oxidants, such as the
poss如lity to change the ratio of the H2S04/H心2 mixture or to replace part of H2S04 with HN03 as an electrolyte. GDYO
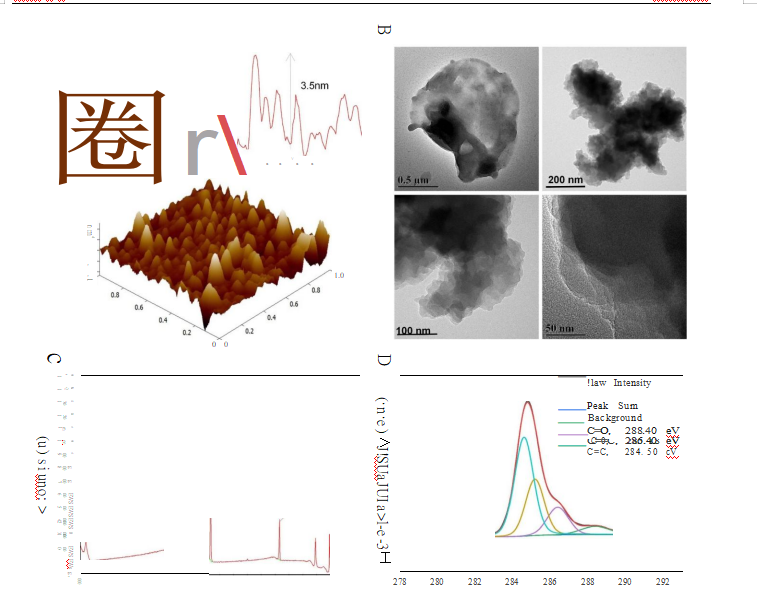
used in this research was produced via a modified Hummers'method.36--38 The morphological features of GDYO were determined in AFM images, and we found that the average height of GDYO was 3.5 nm (Figure IA). TEM suggested that GDYO displays a nanosheet-like morphology(Figure I B). The XPS (Figure IC and Figure I) spectrum of the GDYO nanosheet shows a peak at 284.4 eV, which corresponds to the binding energy of the C ls orbital, and four subpeaks ofC ls at 284.5, 285.2, 286.4, and 288.4 eV, which are attributed to the orbitals of C=C (sp2), C式(sp), C-0, and C=Obonds, respectively, implying the effective linkage of the benzene ring and two acetylenic linkages. The Raman spectrum ofGDYO is
shown in Figure IE, with prominent peaks D and G at 1371 cm-1 and 1560 cm飞respectively, corresponding to carbon
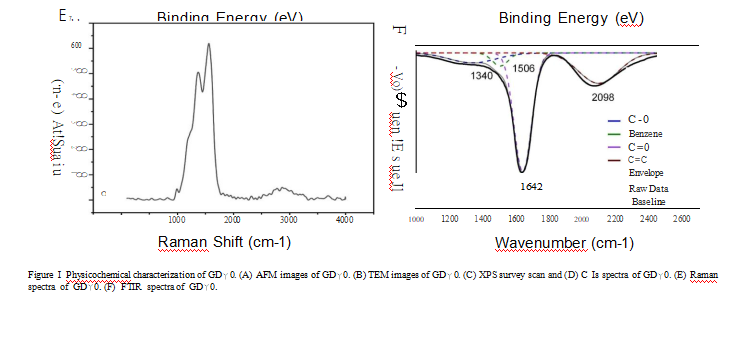
materials. The FTJR spectrum ofGDYO shows a peak at 1506 cm-1 ascribed to the benzene ring; a peak at 1642 cm-1 ascribed to C=O stretching vibration; and a peak at 2098 cm-1 ascribed to C式stretching vibration (Figure lF).
For in vitro treatments, a CCK-8 assay was perfonned to examine the cytotoxicity of GDYO. As shown in Figure 2C, GDYO exhibited no significant cytotoxicity toward NOK cells, even when the concentration ofGDYO reached 100 µg/mL, demonstrat ing the good biocompat伽lity ofGDYO, consistently with previous results. Therefore, it can be assumed that GDYO has some advantages in biological applications, owing to its superior biocompat伽lity and low toxicity. In contrast, upon laser irradiation, the viability ofSCC9 cells showed obvious dose-dependent PDT efficiency along with an increase in the concentration ofGDYO (10 ug/mL-150 ug/mL). The cell viability decreased from 89% to 37.4%, highlighting its excellent performance as
Figure 2 In vitro anticancer efficacy. (A) Antitumor effects of G研0-PDT in SCC9 cells stained with Pl (red, dead cells) and calcein AM (green, live cells), respectively Fluorescent images were obtained by fluorescence microscopy. Scale bar= I 00 nm. (B) Relative fluorescence quantitative analysis of live/dead fiuorescence microscopy. (C) Cell viability of GD丫0 at different concentrations in the NOK normal cell line. (D) Cell viability of SCC9 cells incubated with G饮0 at different concentrations under irradiation. (E) Confocal microscopic images of SCC9 cells after exposure to GD丫0 for 6 h. Scale bar = IS µm. **P<0.0 I, ***P<0.00 I, ****P <0.000 I
电话
微信扫一扫
